Free Shipping over $199 -
Use new customer discount code NEWCUST at checkout for 10% off your entire order!
Popular Products
Bacteriostatic Water for Injection USP 30mL (single bottle)
- $15.95
$19.99- $15.95
- Unit price
- / per
* Limited Inventory as of December 2025 Expiration Date: 31 FEB 2027 Brand: Hospira Dispensed in a 30mL, plastic, multi-dose vial. Designed solely for parenteral use (drug administration by intravenous, intramuscular, or subcutaneous injection) following the addition of drugs that require dilution or must...
- $15.95
$19.99- $15.95
- Unit price
- / per
Bacteriostatic Water for Injection USP 30mL (3 pack)
- $41.85
- $41.85
- Unit price
- / per
Expiration Date: 31 FEB 2027 Brand: Hospira Dispensed in a 30mL, plastic, multi-dose vial. 3 bottles total. Designed solely for parenteral use (drug administration by intravenous, intramuscular, or subcutaneous injection) following the addition of drugs that require dilution or must be dissolved in an...
- $41.85
- $41.85
- Unit price
- / per
Bacteriostatic Water for Injection USP 30mL (5 pack)
- $67.75
- $67.75
- Unit price
- / per
Expiration Date: 31 FEB 2027 Brand: Hospira Dispensed in a 30mL, plastic, multi-dose vial. 5 bottles total. Designed solely for parenteral use (drug administration by intravenous, intramuscular, or subcutaneous injection) following the addition of drugs that require dilution or must be dissolved in an...
- $67.75
- $67.75
- Unit price
- / per
Shipping Notifications: Orders ship Monday - Friday until 2 PM (CST).
SKU:
EXE 26016|case
Availability:
In Stock
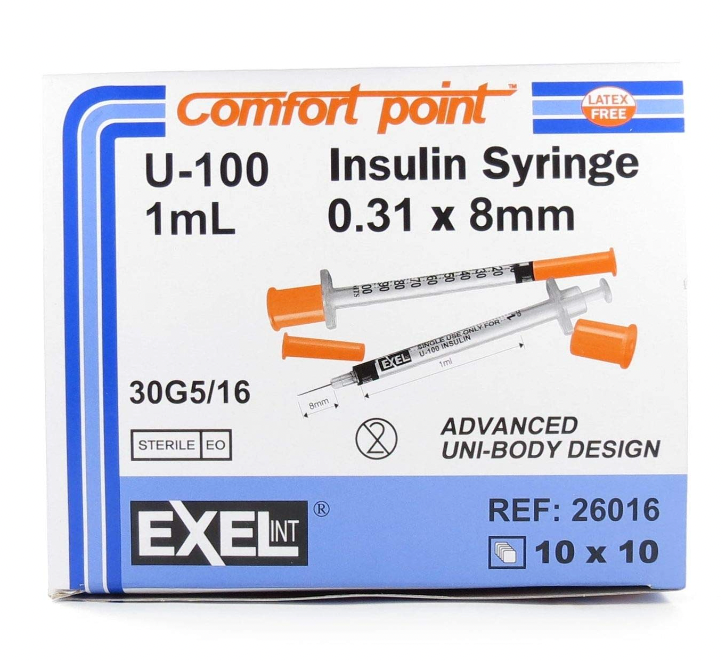
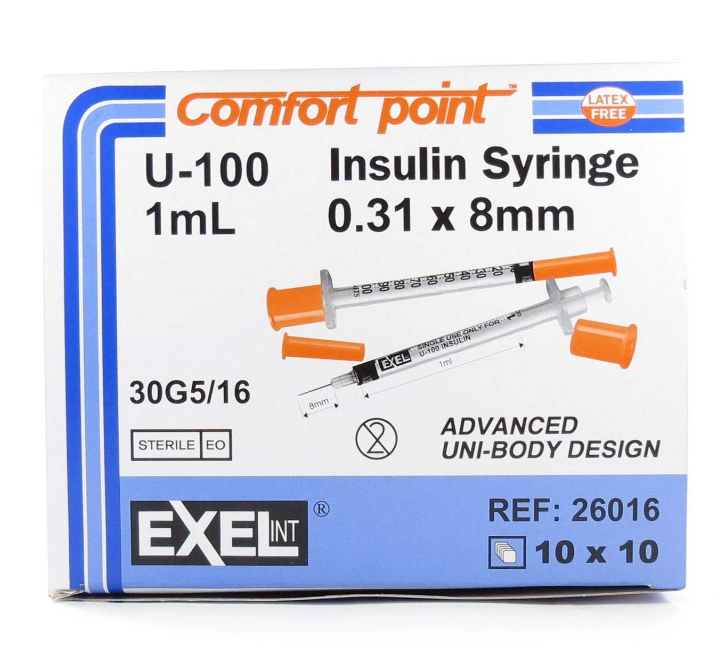
Exel U-100 ComfortPoint 1ml Insulin Syringe 30G x 5/16" (BY CASE)
- $137.15
- $137.15
- Unit price
- / per
Features for Product No: 26016 Sterile, Non-Toxic, Non-Pyrogenic Permanently attached needle Reduced dead space for reduced insulin waste Smooth...
Subtotal:
$137.15
10 customers are viewing this product
Free Shipping
Free standard shipping on orders over $199
SHIPPING NOTIFICATION:
Holiday Shipping Schedule — 2025
Peak-season volumes may delay regular ground shipments. Time-definite services and pickup dates are below.
Service Alerts
- Ground Advantage is currently 3–5 business days.
- Delivery dates shown are ESTIMATED, not guaranteed.
- For guaranteed delivery, select UPS Next Day Air® or USPS Priority Express.
- Saturday delivery available in certain locations only.
Note: USPS Ground Advantage can take 7–9 business days to some ZIP codes—choose expedited shipping if you’re on a deadline.
Thanksgiving
Note: Due to “peak season”, regular ground shipping may experience delays.
- Wednesday, Nov 26 — All UPS Next Day Air® and USPS Priority Express Mail picked up Nov 26 are scheduled for delivery on Friday, Nov 28.
- Wednesday, Nov 26 — UPS 2nd Day Air® picked up Nov 26 is scheduled for delivery on Monday, Dec 1 (except those processed/labeled for Saturday, Nov 29 — additional fee required).
- Thursday, Nov 27 — CLOSED
- Friday, Nov 28 — CLOSED
Christmas
Note: Due to “peak season”, regular ground shipping may experience delays.
- Monday, Dec 22 — Last day to ship UPS 2nd Day Air® for delivery on Wednesday, Dec 24. UPS 3 Day Select® picked up Dec 22 is scheduled for delivery on Friday, Dec 26.
- Tuesday, Dec 23 — Last day to ship UPS Next Day Air® for delivery on Wednesday, Dec 24.
- Wednesday, Dec 24 (Christmas Eve) — CLOSED
- Thursday, Dec 25 — CLOSED
Description
xFeatures for Product No: 26016
- Sterile, Non-Toxic, Non-Pyrogenic
- Permanently attached needle
- Reduced dead space for reduced insulin waste
- Smooth plunger action for greater filling accuracy
- Auto ground tip for greater comfort
- This product does not contain Natural Rubber Latex
1 case - 5 boxes - 10 bags | 100 syringes per box
Shipping Weight: 5.36 lbs.
Dimensions: Length: 17 in x Width: 7 in x Height: 6 in
Specifications vary slightly between sizes.
Specifications vary slightly between sizes.
Packaging subject to change.
Related Products
Liquidations Outlet
Example product title
- $137.15
- $137.15
- Unit price
- / per
Liquidations Outlet
Example product title
- $137.15
- $137.15
- Unit price
- / per
Liquidations Outlet
Example product title
- $137.15
- $137.15
- Unit price
- / per
Liquidations Outlet
Example product title
- $137.15
- $137.15
- Unit price
- / per
Liquidations Outlet
Example product title
- $137.15
- $137.15
- Unit price
- / per
Liquidations Outlet
Example product title
- $137.15
- $137.15
- Unit price
- / per
Liquidations Outlet
Example product title
- $137.15
- $137.15
- Unit price
- / per
Liquidations Outlet
Example product title
- $137.15
- $137.15
- Unit price
- / per
Liquidations Outlet
Example product title
- $137.15
- $137.15
- Unit price
- / per
Liquidations Outlet
Example product title
- $137.15
- $137.15
- Unit price
- / per
- Choosing a selection results in a full page refresh.